
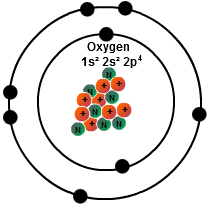
Oxygen is colorless, odorless, and tasteless in its gaseous form, and condenses to pale blue liquid and solid forms. The initial two electrons in the electron configuration for oxygen will be in the 1s orbital. Accounting for one-fifth of the earth’s atmosphere, oxygen combines with most elements and is a component of thousands of organic compounds. In a single oxygen atom, there are eight protons, eight electrons, and eight neutrons. Oxygen is critical for life on Earth, produced by plants during photosynthesis and necessary for aerobic respiration in animals.
#Oxygen electrons how to
We’ll also look at why Oxygen forms a 2- ion and how the electron configuration for. How to write oxygen electron configuration Oxygen contains a total 8 number of electrons in its two electronic shells (K and L). Oxygen, the "elixir of life", was discovered by Joseph Priestly and Carl Wilhem Scheele independently of each other in the 1770’s. 722 108K views 3 years ago In this video we will write the electron configuration for O 2-, the Oxide ion. It is produced by plants during photosynthesis.
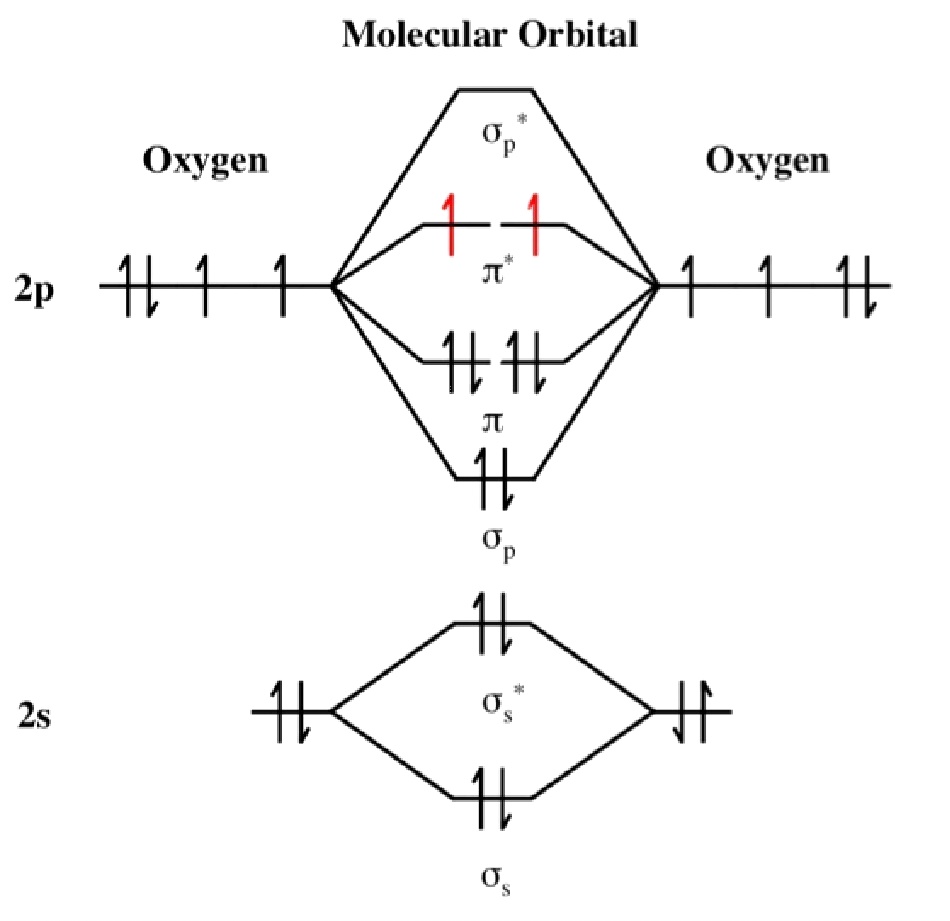
The credit for discovering oxygen is now shared by three chemists: an Englishman, a Swede, and a Frenchman. This was oxygen although it was not identified as such. It is one of the two major components of air. Elements and Periodic Table History In 1608, Cornelius Drebbel had shown that heating saltpetre (potassium nitrate, KNO 3) released a gas. It is the most common component of the earth's crust at 49. Alone, each oxygen atom has six valence electrons. It is the third most abundant chemical element in the universe after hydrogen and helium. Look at the oxygen atoms in the figure above. A more electronegative element wouldn't necessarily have any effect on the rate of electron flow down the ETC and therefore wouldn't affect the rate of ATP production.Discoverer: Joseph Priestley/Carl Scheele Water (H 2 O) Oxygen difluoride (OF 2) Interesting facts. P.S remember oxygen is not producing the ATP itself it is merely keeping the transport chain unblocked so the electrons keep flowing. If you need to write For example, we know that. Please bear in mind these are just my thoughts. How to Write an Electron Configuration Looking at the periodic table, you can see that Oxygen has 8 electrons.
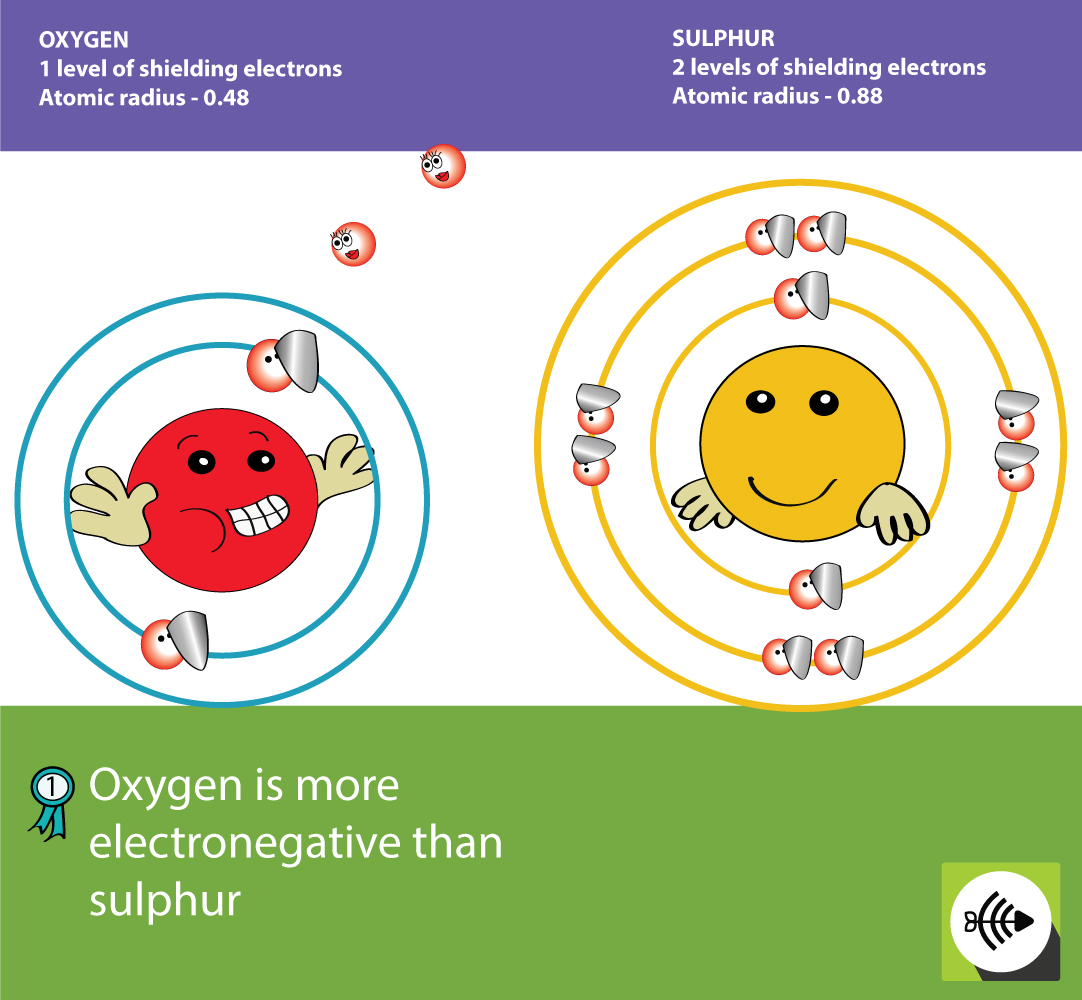
Finally fluoride is known to be damaging to the body above certain concentrations affecting things like the nervous system and hormone secretion as well as protein synthesis. Also if fluorine were used as the terminal electron acceptor it would form HF, hydrofluoric acid in solution which is hard for the cells to deal with and would affect pH in the cytosol affecting enzyme function whereas oxygen just forms water. In addition fluorine is very reactive so would not exist by itself for very long. Oxygen makes up 21% of our atmosphere and is stable in both air and water whereas fluorine is much rarer. Through reduction, oxygen atoms are removed close to the interface, leaving behind oxygen vacancies in the SrTiO3 lattice and mobile electrons in the SrTiO3. The first is simply to do with availability. There are a few reasons that spring to mind.


 0 kommentar(er)
0 kommentar(er)
